Cleanroom Validatie Engineer

Cleanroom Validatie Engineer (Nederlands) Bilzen (HQ), Belgium Voltijds Over de job Als cleanroom validatie engineer voor onze klanten in België en Nederland beschik je over een mix van multidisciplinaire vaardigheden. Deze vaardigheden variëren van het toepassen van je technische kennis op het gebied van cleanroomtechnologie, tot het onderhouden, repareren en valideren van onze cleanrooms. Je […]
Cleanroom Technieker

Test
Projectmanager Cleanrooms

Projectmanager Cleanrooms Bilzen (HQ), Belgium Voltijds Over de job Als Cleanroom Project Manager ben je betrokken bij het ontwerp, de realisatie en het onderhoud van complexe uitdagingen in laboratoria, ziekenhuizen, biotech & pharma, voeding, onderzoek en hightech bedrijven. Je bent verantwoordelijk voor het beheer, de coördinatie en succesvolle afronding van onze Cleanroom projecten in de […]
ABN Cleanroom Technology configures and constructs VIX-cleanroom facility for Wilting

ABN Cleanroom Technology is excited to announce a new 650m? cleanroom project in the Netherlands for Wilting Components BV. Wilting is located in Eindhoven and specialises in the production of high-value precision industry components and serves several European OEM companies that are active in high-tech industries.
ABN Cleanroom Technology invests in antibody production

Limburg-based companies ABN Cleanroom Technology and simAbs are joining forces to make great strides together within the Life Sciences sector. ABN Cleanroom Technology will provide simAbs, which is currently housed at Bioville Health Campus, with a cGMP-certified cleanroom that meets all requirements to run their operations and increase their production capacity.
WHITEPAPER | 5 Forces that drive energy efficiency in modular cleanroom design

Cleanrooms consume large amounts of energy compared with the energy consumption in non-classified rooms, like commercial buildings. In this whitepaper, you will learn 5 important forces that are inherent to the design of energy efficient cleanrooms
DryCell – The first ready to use low dewpoint cleanroom solution in EU
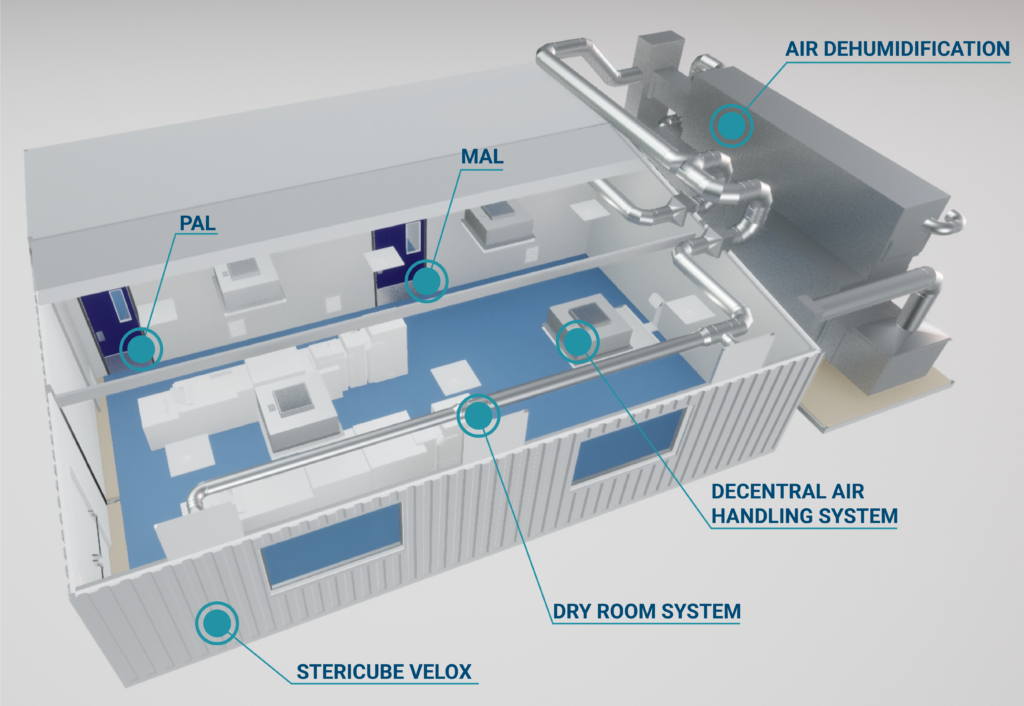
With the increasing demand for electronic devices and electric vehicles throughout the world, more and more manufacturers are running into the same problem: the supply of components, especially batteries, is not keeping up with the massive demand. To meet overall demand in time, additional facilities for production of lithium-ion batteries should be built in the short-term. Battery cell production is a mission critical challenge because the materials used in lithium-ion batteries react heavily with humidity. For that reason, we introduced DryCell.
Digitizing GMP validation processes

A company?s records are a prized possession and should be taken care of the best way possible. When we think of?good manufacturing practices (GMP), regulations must be followed to keep the quality and integrity of these records in good standing.?Records are official evidence proving that certain tasks have been completed as they should be ?evidence of compliance? and poorly documenting can negatively impact cleanroom process quality. In this article, we?ll shortly discuss paper records vs. electronic records for GMP processes.
The rise of prefabricated cleanroom solutions for the world to come

With more and more stringent regulatory standards, developing regions that offer growth opportunities and a higher demand for mechatronics & pharmaceuticals, the number of requested cleanrooms in the field keeps increasing.
Predictive maintenance: The holy grail in a pre-engineered cleanroom environment
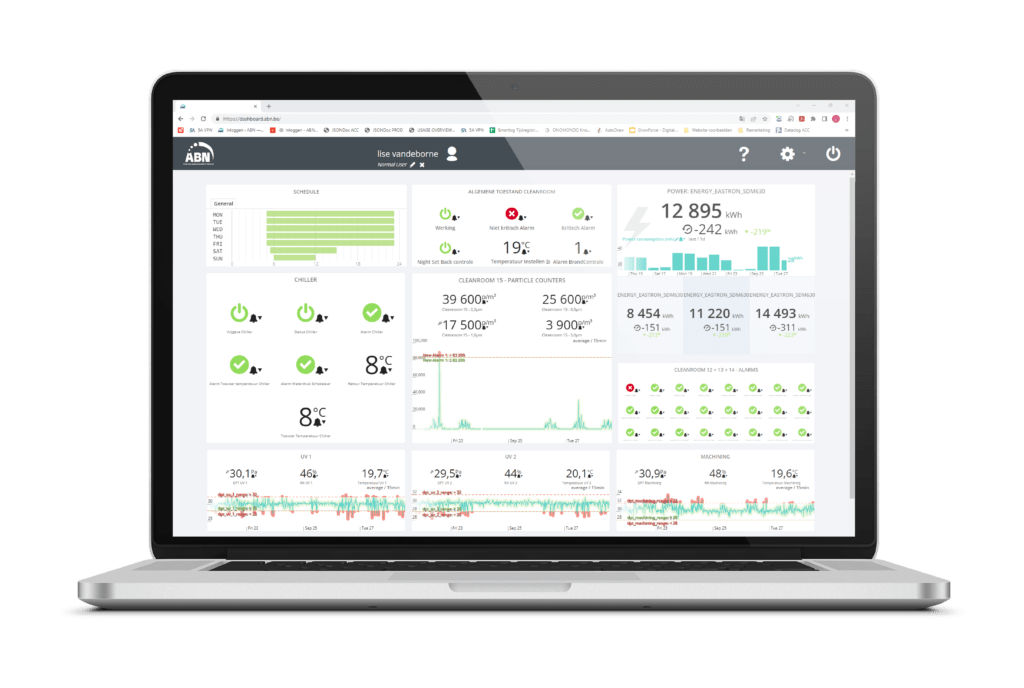
Cleanroom sensor technology, the Internet of Things and big data: these developments are the enablers for modern-day predictive maintenance in pre-engineered cleanroom design.
