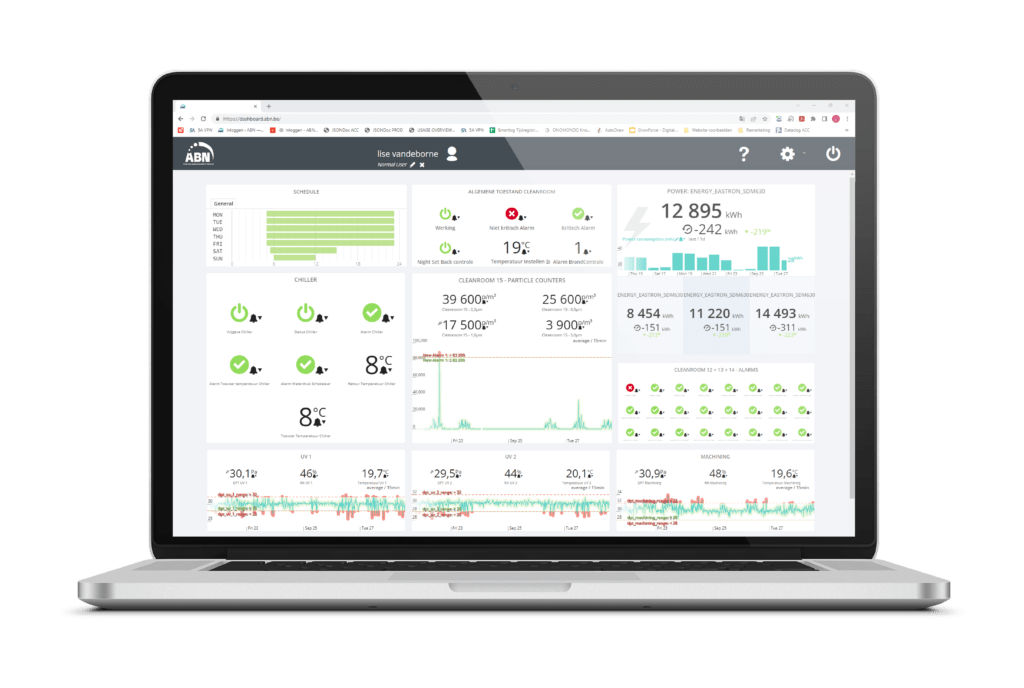
By using our predeveloped, open and GMP compliant IoT cloud platform for cleanroom environments, we are able to set alert limits in an easy way and be sure that when a potential problem occurs, we will catch it with an alert rule. The smart rules wizards that we use are flexible enough to cover several alert limits – high alert, medium alert and low alert – and additional parameters like alerting delay configured per measurement requirements.
As a cleanroom supplier with strong focus on the Cleanroom-As-A-Service model, the role of smart notifications in pre-engineered cleanroom environments is extremely important. By offering our customers a platform for data gathering, data visualization and alarm management, we help them create a cleanroom environment with a notification system where you decide what you want to be notified about.
GMP Connect key features:
GMP Connect is a Software-as-a-Service (SaaS) platform, you only pay for what you use. We allow you to focus on what matters most to you while we take care of all the technical things like server configurations, cloud deployment, hosting, validation and data back-ups.
GMP Connect can interact with other external systems. A validated cloud environment for GMP use will only be valid when using the software from ABN. Data extraction to another system can be done via an API integration, but will never replace ABN’s GMP Connect Cloud Platform. Our validtion engineers will never validate external software for GMP use.
The license fee includes online support, cloud hosting, built-in back-up services, user manual, training material and all future updates of the current software modules with a change control mechanism that allows the user to remain in a validated state continuously. Additionally, GMP Connect provides customer support and validation documentation for Installation Qualification and Operational Qualification. An alarm management module with notifications to the authorized users as well as rights & roles management are included in the license fee.
GMP Connect is designed to be a web-based interface as this is the most scalable and secure option. No software needs to be installed on local desktops. You can access your cleanroom 24/7 from any location as long as you have mobile network connectivity. The data is stored on AWS.
Implementing a CFR21 Part 11 compliant cloud environment requires the necessary documentation, user specifications and validation period. The time to implement and validate depends on the size of your cleanroom area that requires a GMP compliant cloud platform.
Do you want to receive more information about how pre-engineered building blocks and circular cleanroom design will benefit your project? Simply send over a request. You can also contact us with questions about our services, ranging from cleanroom validation and GMP-monitoring, to commissioning and cleanroom remodeling.
By using our predeveloped, open and GMP compliant IoT cloud platform for cleanroom environments, we are able to set alert limits in an easy way and be sure that when a potential problem occurs, we will catch it with an alert rule. The smart rules wizards that we use are flexible enough to cover several alert limits – high alert, medium alert and low alert – and additional parameters like alerting delay configured per measurement requirements.
As a cleanroom supplier with strong focus on the Cleanroom-As-A-Service model, the role of smart notifications in pre-engineered cleanroom environments is extremely important. By offering our customers a platform for data gathering, data visualization and alarm management, we help them create a cleanroom environment with a notification system where you decide what you want to be notified about.
GMP Connect key features:
GMP Connect is a Software-as-a-Service (SaaS) platform, you only pay for what you use. We allow you to focus on what matters most to you while we take care of all the technical things like server configurations, cloud deployment, hosting, validation and data back-ups.
GMP Connect can interact with other external systems. A validated cloud environment for GMP use will only be valid when using the software from ABN. Data extraction to another system can be done via an API integration, but will never replace ABN’s GMP Connect Cloud Platform. Our validtion engineers will never validate external software for GMP use.
The license fee includes online support, cloud hosting, built-in back-up services, user manual, training material and all future updates of the current software modules with a change control mechanism that allows the user to remain in a validated state continuously. Additionally, GMP Connect provides customer support and validation documentation for Installation Qualification and Operational Qualification. An alarm management module with notifications to the authorized users as well as rights & roles management are included in the license fee.
GMP Connect is designed to be a web-based interface as this is the most scalable and secure option. No software needs to be installed on local desktops. You can access your cleanroom 24/7 from any location as long as you have mobile network connectivity. The data is stored on AWS.
Implementing a CFR21 Part 11 compliant cloud environment requires the necessary documentation, user specifications and validation period. The time to implement and validate depends on the size of your cleanroom area that requires a GMP compliant cloud platform.

Do you want to receive more information about how pre-engineered building blocks and circular cleanroom design will benefit your project? Simply send over a request. You can also contact us with questions about our services, ranging from cleanroom validation and GMP-monitoring, to commissioning and cleanroom remodeling.
Legolisation means standardisation. Standardisation causes a shift in production. Work is carried out in conditioned spaces such as factory halls. our cleanrooms are manufactured partly or entirely off-site, which means huge savings on transport costs and reduction of inconvenience on-site